Manage soil acidity – South Coast Sandplain WA
The issue – Soil acidity
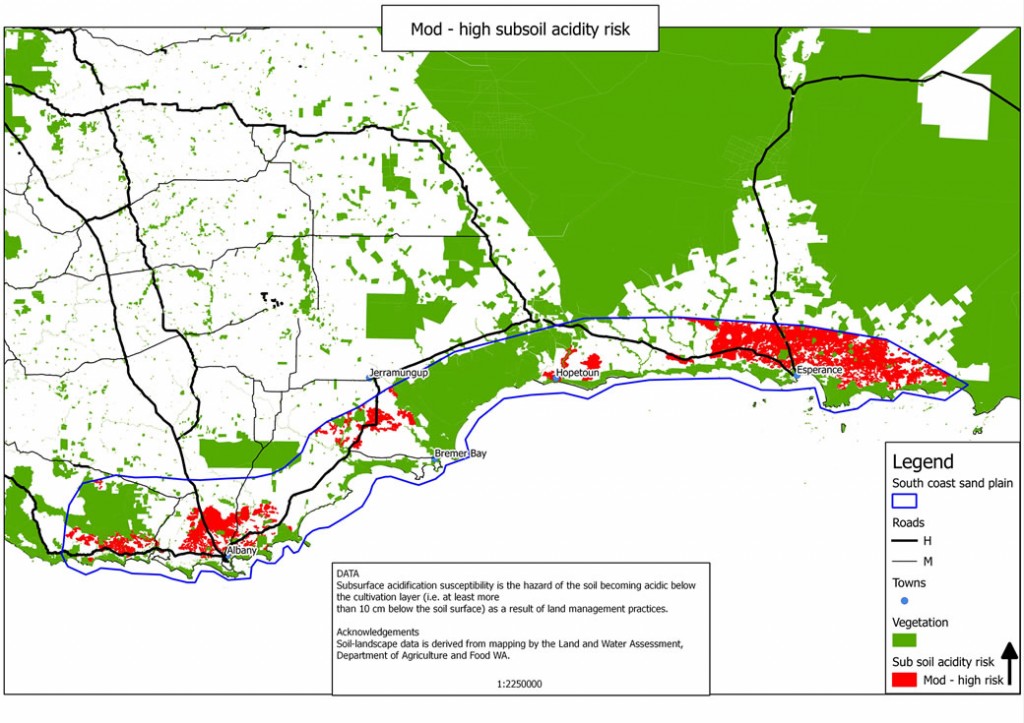
Sub-soil acidity is one of the biggest issues in the South Coast Sandplain zone with approximately 979,000ha or 32% of the region at moderate to high risk (DAFWA Resource Management Technical Report 2008).
Soil acidification can affect all layers of the soil. However, sub-soil acidification (below the top 10cm) is perhaps one of the more difficult issues to treat in the short term. It has a direct impact on the productivity of pastures and crops as well as affecting the sustainability of the resource base. There are also off site impacts due to the effects on waterways (soil and nutrient loss to waterways), ground water pollution (leaching of nitrates) and salinity (reduced water use efficiency of crops and pastures) (DAFWA Bulletin 4784, 2009).
The cause – product removal and nitrogen
[expand title=” ” swaptitle=” ” tag=”h4″ elwraptag=”div” elwrapclass=”expend-collapse-wrapper”]
Soil acidification is in many ways a natural process that has been accelerated by the activities of agriculture. Processes around product removal, the cycling of nitrogen and to a lesser extent the build up and cycling of organic matter all contribute to rates of acidification (DAFWA Bulletin 4343 1998)
A significant contributor to soil acidification is product removal. Plants will tend to excrete hydrogen ions to allow the uptake of positively charged nutrients. These excess hydrogen ions are then often re-absorbed resulting in no real change. However, if the plant is harvested, this will result in the removal of cations (positively charged nutrients) in the plant material and will leave the hydrogen ions in the soil, causing acidity. Cutting hay is perhaps one of the worst crops for causing this problem because of the amount of biomass removed. Whether it is cutting hay, harvesting a crop or grazing pasture, some acidification will generally occur due to product removal.
The other big contributor is nitrogen. Whether it is applied nitrogen or nitrogen from legumes, there is the potential for acidification because of the loss of nitrogen through leaching. If the nitrogen cycling is able to occur without this leaching, the possibility of acidification is greatly reduced.
During the cycling of nitrogen in the soil, nitrate is produced, which is highly mobile and easily leached. If this occurs, cations are usually carried with it leaving behind hydrogen ions and acidifying the soil. This can occur with both legumes and applied nitrogen fertilisers. Ammonium based fertilisers are a little different and will acidify the soil even without leaching, which is different to the more common forms of nitrogen fertilisers like urea.
There is some evidence that high levels of organic matter can also cause increased acidification. This is dependent on a number of factors including the pH to begin with. It is not always the case that this will occur, however it is very possible that high organic matter levels will increase the soils buffering capacity, making it harder to shift the pH.
For further information on the causes of soil acidification refer to DAFWA bulletin 4343, Soil Guide – A handbook for understanding and managing agricultural soils.
[/expand]
Benefits of correct pH balance
[expand title=” ” swaptitle=” ” tag=”h4″ elwraptag=”div” elwrapclass=”expend-collapse-wrapper”]
One of the most significant benefits to pasture systems is the mobilisation and more efficient uptake of macro nutrients. Nutrients like nitrogen (N), potassium (K), phosphorous (P) and calcium (Ca) all increase in availability with rising pH (Fig 2). This means that if soils are acidic (below key thresholds), less of the applied nutrients will be taken up. By raising soil pH to key levels it is possible to increase the efficiency of fertiliser utilisation.
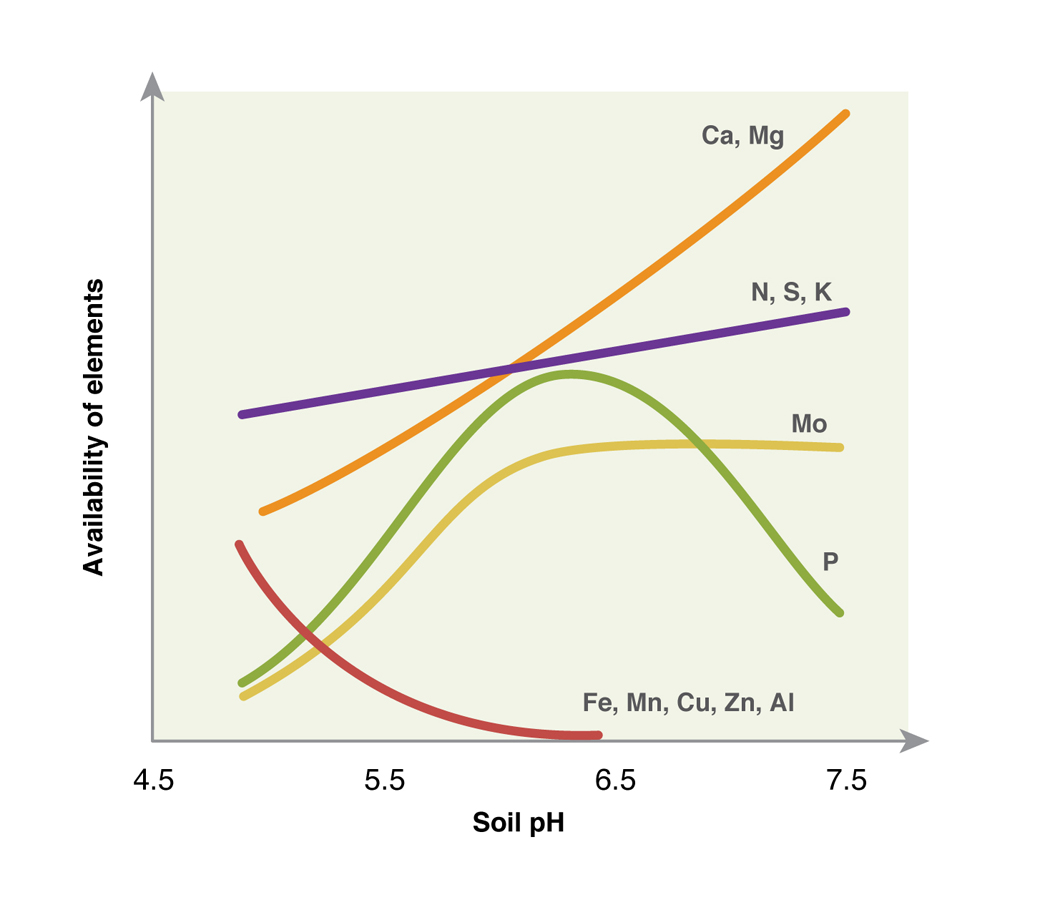
The other significant factor of an acidic soil is the availability of aluminium (Al). It is a micronutrient that will increasingly move into soil solution in acidic soils (below 5.5). If aluminium toxicity occurs it is likely that the growth of pasture plants, especially clover will be affected. The only way to avoid this is to keep soil pH above the key threshold of 5.5 in the top soil and 4.8 for subsoils.
There are other benefits which can be attributed to soil acidity which include:
- Better composition in pastures, especially clover,
- More vigorous growth,
- More efficient utilisation of soil water, and
- Better conditions for biology activity.
Diagnosing soil acidity
[expand title=” ” swaptitle=” ” tag=”h4″ elwraptag=”div” elwrapclass=”expend-collapse-wrapper”]
It is not always possible to infer the subsoil pH from the surface pH. As a result it is crucial that samples are taken from the 0-10cm, 10-20cm and if possible the 20-30cm depths before a large investment is made in buying and applying lime.
Sampling errors and lack of representative samples can lead to very misleading test results. To avoid this there are some simple ‘must do’s’ associated with soil samplings, these are:
- Always sample in summer when the soil is dry,
- Scrape away the top organic matter and if possible put the sample through a 2mm sieve to remove gravel, organic matter and other large foreign material,
- Paddock variability should be taken into account and samples grouped to major soil types/land management units,
- Take at least 30 subsamples per soil unit or paddock in a zig zag pattern, mix these and take your subsample for analysis,
- Avoid atypical areas (fertiliser or lime dumps, headlands, fire breaks etc),
- Use clean equipment (not rusty or used to shovel super),
- Store in clean dry bags or containers,
- Use a reputable lab with NATA (National Association of Testing Authorities, Australia) accreditation with WA calibration curves,
- Ensure the test methodologies are suited to WA soils (Colwell P and K, pH in CaCl etc), and
- Avoid swapping labs if possible.
The options for management of soil acidity
[expand title=” ” swaptitle=” ” tag=”h4″ elwraptag=”div” elwrapclass=”expend-collapse-wrapper”]
The following information is brief summary of the main points for the management of surface and sub-surface acidity. More detailed information on the options for managing soil acidity can be found in DAFWA bulletin 4784, 2009. It outlines the management, chemistry and biology of soil acidity and the use of Agricultural lime. Additional resources including a lime comparison calculator can be found on the Soil Quality Website. Information on the quality of various lime pits in WA can be found on the Lime WA Incorporated website.
[/expand]
Liming acidic soils
[expand title=” ” swaptitle=” ” tag=”h4″ elwraptag=”div” elwrapclass=”expend-collapse-wrapper”]
The key targets for pH (CaCl) are 5.5 in the top soil (0-10cm) and 4.8 in the subsoil. These targets will treat ongoing soil acidification and will allow for the movement of carbonates down the profile to treat subsurface pH.
Table 1: Rule of Thumb guides for 100%NV lime applied over 5 yrs (DAFWA bulletin 4784, 2009)
| Soil Depth | pH (CaCl) | Lime amount over 5 years |
| 0-10cm | <5, <5.5 | 2t/ha, 1t/ha |
| Plus | ||
| 10-20 | <4.5, <4.8 | 2t/ha, 1/t/ha |
| Plus | ||
| 20-30 | <4.5, <4.8 | 1t/ha, Measure in 3yrs |
Rates for lime based on the critical pH targets are identified in Table 1. The important consideration is that these rates are over five years and that they are using 100% NV or neutralising value lime. Actual lime rates will vary according to the neutralising value of the lime and its coarseness.
There are many sources of lime and considerable differences in the quality and it is important that producers account for this when considering where to buy lime and what rates to apply.
The important thing to note is that the amount of lime you will need to apply will be based on the recommended rate and the percentage efficiency or effective neutralising value (ENV). The table below demonstrates how to calculate the ENV. This can then be used to determine the rate you need to apply and which is the more cost effective. The Soil Quality website has a calculator that will determine the most cost effective lime for your situation based on the fineness of the lime, neutralising value
Table 2: Calculation of percent efficiency based on lime quality analysis (DAFWA bulletin 4784, 2009)
| Particle size (mm) | Particle size discount figure | % of lime* | Neutralising value* | Percent Efficiency (PE) |
| 0 – 0.125 | 1 | 5 | 90 |
(5/100) X 90 X 1 = 4.5 |
| 0.125 – 0.25 | 1 | 48 | 90.5 |
(48/100) X 90.5 X 1 = 43.4 |
| 0.25 – 0.5 | 1 | 38 | 94.8 |
(38/100) X 94.8 X 1 = 36 |
| 0.5 – 1 | 0.5 | 8 | 72.1 |
(8/100) X 72.1 X 1 = 2.9 |
| >1 | 0.2 | 1 | 62.5 |
(1/100) X 62.5 X 1 = 0.1 |
|
SUM = 86.9 |
||||
*Should be based on information from your chosen lime pit.
The rate can be calculated with the summed percent efficiency using the following calculation:
- 100/PE = rate e.g. 100/86.9 = 1.15t/ha of example lime per tonne of 100% neutralising value lime
Producers should aim to access the highest neutralising value lime that is the most cost effect for them. The comparative cost of the lime can be calculated with the percent efficiency however the calculator on the Soil Quality website will do this for you.
[/expand]
Complementary strategies
[expand title=” ” swaptitle=” ” tag=”h4″ elwraptag=”div” elwrapclass=”expend-collapse-wrapper”]
It is possible to adopt strategies that limit the acidification of the soil in some circumstances. The type and application of nitrogen is a one area that can have an impact. The following strategies can be useful, particular if the soil is already acidic:
- Try to apply nitrogen when plants are actively growing,
- Split applications of nitrogen,
- Avoid wet waterlogged areas, and
- Avoid acidifying forms of nitrogen (ammonium based fertilisers).
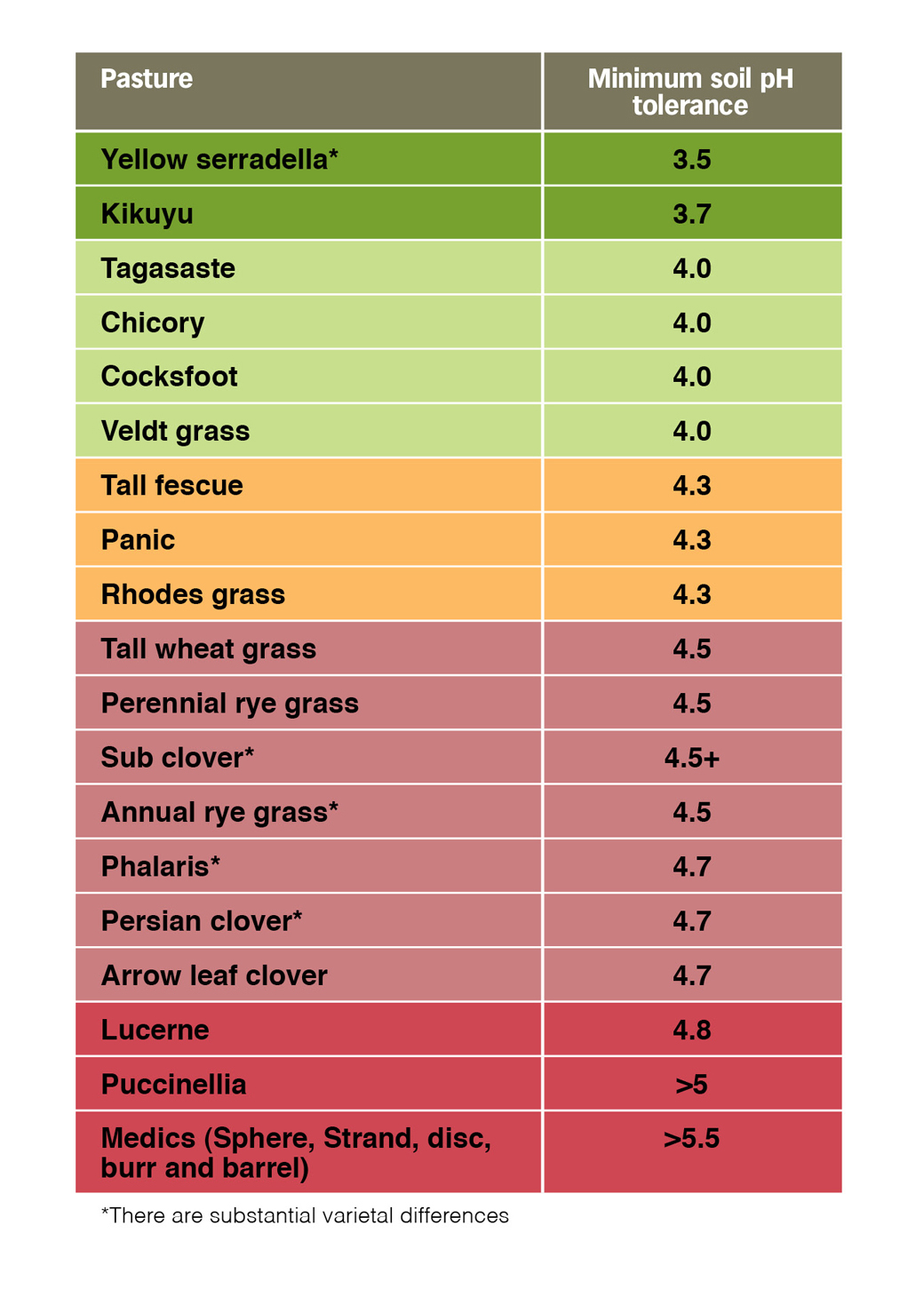
The other strategy is to plant crops and pastures that are more tolerant of soil acidity. This does not preclude the use of lime however it may stabilise production while lime is applied. Generally speaking cereal ryes, triticiale, lupins, oats and serradella are highly to moderately acid tolerant while lucerne, medics, barley, canola and sub clover are highly to moderately sensitive to soil acidity. There are substantial varietal differences within crops that need to be considered.
Table 3 outlines the acid tolerance of a range of perennial and annual pastures. While there are several perennial plants that are highly to moderately acid tolerant, many of the annual legumes are not. This is particularly important for kikuyu which will tolerate highly acidic soils. If the legume component is affected by subsoil acidity it may struggle to persist effecting winter and summer production.
Table 3: Acid tolerance of common perennial pasture, annual legume species and ryegrass